July 13, 2023 by MMR
birth control pill, FDA, Food and Drug Administration, Opill, oral contraceptive
Leading Headlines, Retail News, Topics

WASHINGTON — The Food and Drug Administration on Thursday approved the oral contraceptive Opill for over-the-counter sales, making it the first hormonal contraceptive pill available in the U.S. without a prescription. The approval marks a major win for medical groups, including the American Medical Association and the American College of Obstetricians and Gynecologists, which have
February 15, 2023 by smeyer and MMR
Consumer Healthcare Products Association, Consumer Healthcare Products Association (CHPA), FDA, naloxone
Leading Headlines, Retail News, Supplier News, Topics

WASHINGTON — The Consumer Healthcare Products Association (CHPA) released the following statement in advance of FDA Advisory Committee meetings considering OTC access to naloxone: “Over-the-counter (O-T-C) availability of naloxone – a life-saving emergency therapy for opioid overdoses that is currently only available with a prescription (Rx) – would be a critical step forward in public
January 4, 2023 by MMR
abortion pills, CVS, FDA, Walgreens
Leading Headlines, Retail News

NEW YORK — With family planning rights under major threat in several states, the Food and Drug Administration (FDA) announced this week a move to expand access to abortion. Now chains such as CVS and Walgreens will be able to sell abortion pills to those with prescriptions. The FDA gave the green light to mifepristone
December 13, 2022 by MMR
Bausch + Lomb, Biotrue Hydration Boost Contact Lens Rehydrating, FDA
Supplier News

VAUGHAN, Ontario – Bausch + Lomb Corp. announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Biotrue Hydration Boost Contact Lens Rehydrating drops, a preservative-free multi-dose rehydrating drop for use with soft and rigid gas permeable contact lenses. “We are proud to continue to build upon our successful
November 16, 2022 by MMR
FDA, Food and Drug Administration, NGA, Stephanie Johnson, The National Grocers Association (NGA)
Leading Headlines, Retail News

WASHINGTON – The National Grocers Association (NGA), which represents independent supermarket operators, issued a statement on Wednesday contending that a proposed Food and Drug Administration rule on food traceability would disproportionately hurt small grocers. The trade group notes that the proposed rule aims to establish additional traceability recordkeeping requirements for persons who manufacture, process, pack
October 20, 2022 by MMR
FDA, hearing aids, NACDS
Leading Headlines, Retail News

(Left to right): U.S. Commissioner of Food and Drugs, Dr. Robert Califf; Walgreens pharmacist Chuckwuma ‘Chuks’ Onwuka; U.S. Secretary of Health and Human Services Xavier Becerra; and U.S. Senator Elizabeth Warren (D-MA) visit a Walgreens pharmacy in Washington, D.C., on October 19, 2022, as hearing aids go over the counter for the first time. ARLINGTON,
March 29, 2022 by MMR
booster, FDA
Leading Headlines, Retail News, Topics
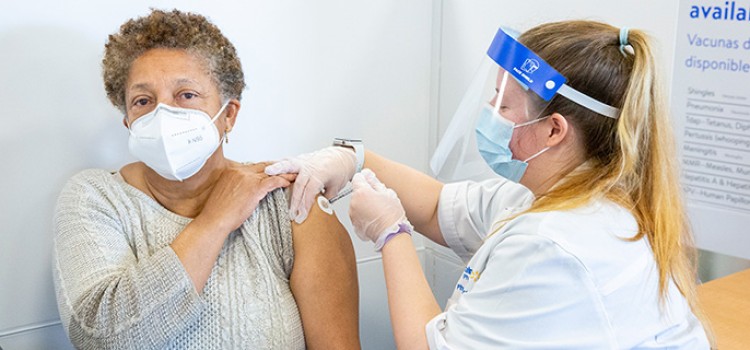
SILVER SPRING, Md. — The U.S. Food and Drug Administration authorized on Tuesday a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for older people and certain immunocompromised individuals. The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series. This action will
January 26, 2022 by MMR
Council for Responsible Nutrition (CRN), CRN, FDA, N-acetyl-L-cysteine (NAC)
Leading Headlines, Supplier News

WASHINGTON — The Council for Responsible Nutrition (CRN), the leading trade association for the dietary supplement and functional food industry, announced Wednesday it filed comments with FDA calling for the agency to respond to its citizen petition on the status of products containing N-acetyl-L-cysteine (NAC). CRN expressed its dissatisfaction with the agency’s most recent response, provided
January 5, 2022 by MMR
Council for Responsible Nutrition, CRN, FDA, N-acetyl-L-cysteine
Leading Headlines, Topics

WASHINGTON — The Council for Responsible Nutrition (CRN), the leading trade association for the dietary supplement and functional food industry, responded Wednesday to FDA’s November 2021 decision not to address CRN’s citizen petition regarding N-acetyl-L-cysteine (NAC) on a timely basis, calling on the agency for swift review of the legal questions CRN raised. Instead, FDA dodged
October 20, 2021 by MMR
CHPA, FDA, OTC hearing aids
Leading Headlines, Topics

WASHINGTON — The Consumer Healthcare Products Association (CHPA) issued the following statement from Marcia Howard, vice president, Scientific and Regulatory Affairs, upon the U.S. Food and Drug Administration (FDA) issuance of a Proposed Rule and Draft Guidance for establishing a regulatory category for over-the-counter (OTC) hearing aids: “CHPA applauds FDA for issuing this long-awaited proposed rule which has the
August 23, 2021 by MMR
BioNTech, COVID-19 vaccine, FDA, Pfizer
Leading Headlines, Retail News, Supplier News, Topics

WASHINGTON — The Food and Drug Administration reported on Monday that it has granted full approval to Pfizer and BioNTech for their COVID-19 vaccine to be given to Americans as young as 16, clearing the way for a wave of moves that health officials say could reverse a nationwide slowdown in the pace of first
November 30, 2020 by Jeffrey Woldt
covid vaccine, FDA, HHS, Jeffrey Woldt
2020, Issue 3-23-2020, Issues
The centrality of retail pharmacies in protecting the health of Americans was underscored earlier this month when the Department of Health and Human Services reached an agreement with a group of national retailers, regional chains and networks of independent drug stores to facilitate COVID-19 immunizations, after one or more vaccines receive Food and Drug Administration










